Baking powder and ammonium carbonate both serve as leavening agents, but baking powder is a modern, compound raising agent that releases carbon dioxide through an acid-base reaction, making it suitable for a wide range of baked goods. Ammonium carbonate, also known as baker's ammonia, is a traditional leavening agent that produces a crisp texture and is ideal for thin, dry baked goods like cookies and crackers due to its strong ammonia smell which dissipates during baking. Choosing between the two depends on the recipe and desired texture, with baking powder offering versatility and ammonium carbonate providing a distinct, crisp finish.
Table of Comparison
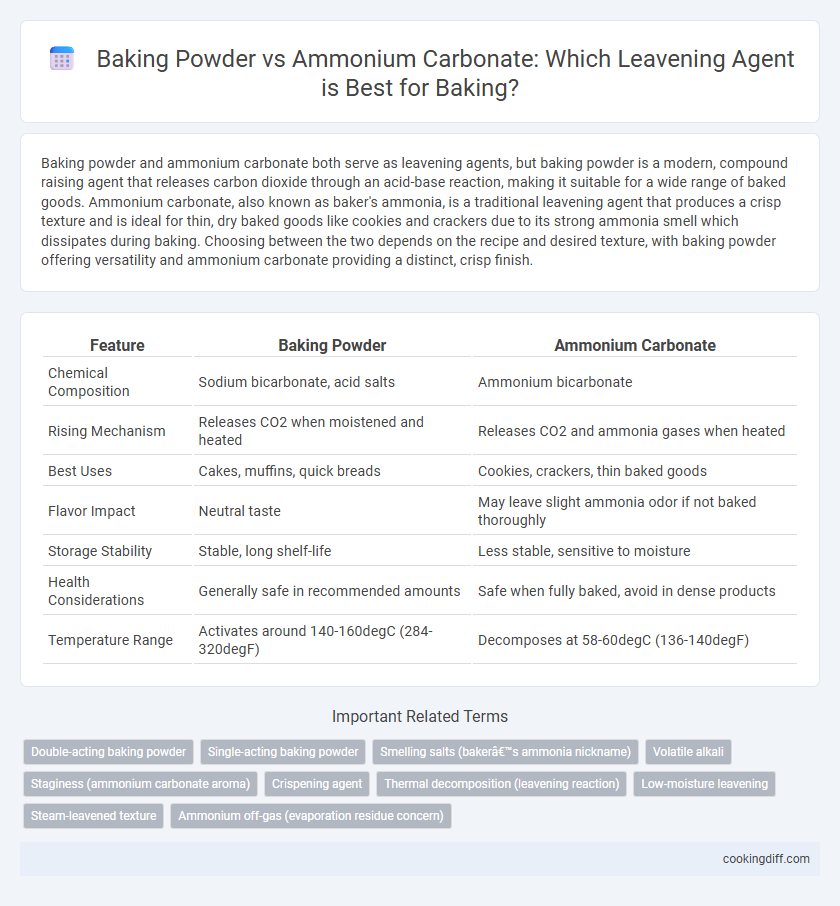
| Feature | Baking Powder | Ammonium Carbonate |
|---|---|---|
| Chemical Composition | Sodium bicarbonate, acid salts | Ammonium bicarbonate |
| Rising Mechanism | Releases CO2 when moistened and heated | Releases CO2 and ammonia gases when heated |
| Best Uses | Cakes, muffins, quick breads | Cookies, crackers, thin baked goods |
| Flavor Impact | Neutral taste | May leave slight ammonia odor if not baked thoroughly |
| Storage Stability | Stable, long shelf-life | Less stable, sensitive to moisture |
| Health Considerations | Generally safe in recommended amounts | Safe when fully baked, avoid in dense products |
| Temperature Range | Activates around 140-160degC (284-320degF) | Decomposes at 58-60degC (136-140degF) |
Introduction to Baking Powder and Ammonium Carbonate
Baking powder is a chemical leavening agent composed of an acid and a base, typically sodium bicarbonate and cream of tartar, which releases carbon dioxide gas when moistened and heated. It is commonly used in cakes, cookies, and quick breads to achieve a light and airy texture.
Ammonium carbonate, also known as baker's ammonia, is an old-fashioned leavening agent that decomposes into ammonia and carbon dioxide when heated, creating a rise in baked goods. It is especially effective in thin, crispy baked items like crackers and certain cookies due to its strong gas release and lack of residual taste.
Chemical Composition: Baking Powder vs Ammonium Carbonate
Baking powder contains a combination of sodium bicarbonate, an acid salt, and a moisture absorber, which react to release carbon dioxide gas for leavening. Ammonium carbonate, also known as baker's ammonia, decomposes into ammonia, carbon dioxide, and water when heated, providing a strong rising effect without residual acidity.
Baking powder is preferred for its balanced chemical composition that offers consistent and controlled leavening suitable for most cakes and quick breads. Ammonium carbonate is favored in traditional recipes like cookies and crackers where a crisp texture is desired, but it can leave an ammonia odor if not fully baked out. Both agents serve as effective leaveners, with baking powder offering moisture stability and ammonium carbonate providing rapid gas release at higher temperatures.
How Each Leavening Agent Works
Baking powder releases carbon dioxide gas through an acid-base reaction when moistened and heated, creating bubbles that cause dough to rise. Ammonium carbonate decomposes upon heating, releasing ammonia and carbon dioxide gases that lighten baked goods rapidly.
- Baking powder contains both acid and base - it activates with moisture and heat to produce steady gas release for even rising.
- Ammonium carbonate breaks down at high temperatures - it releases gas quickly but can leave an ammonia smell if underbaked.
- Baking powder is versatile for various recipes - ammonium carbonate is ideal for crisp, dry products like cookies and crackers.
Ideal Baking Applications for Baking Powder
Baking powder is ideal for quick breads, cakes, and muffins where a consistent and reliable rise is needed without altering the flavor. It reacts with moisture and heat to produce carbon dioxide, giving a light and airy texture suitable for most home baking applications.
- Stable leavening agent - Baking powder provides a controlled release of gas, ensuring uniform rising throughout the baking process.
- Neutral flavor impact - Unlike ammonium carbonate, baking powder does not impart any ammonia-like taste, making it perfect for sweet recipes.
- Moisture-sensitive activation - The double-acting formula causes rising in two stages, enhancing the texture and volume of baked goods.
Baking powder is preferred for batters and doughs requiring delicate crumb structures and balanced flavor profiles.
Best Uses of Ammonium Carbonate in Baking
Ammonium carbonate, also known as baker's ammonia, is ideal for crisp and dry baked goods like cookies and crackers where a light, airy texture is desired. Unlike baking powder, it decomposes completely during baking, releasing ammonia gas that helps create a strong rise without leaving any residue. Its best uses include traditional recipes for old-fashioned cookies, crackers, and some pastries where a clean, crumbly texture is essential.
Impact on Texture, Taste, and Aroma
| Leavening Agent | Impact on Texture | Impact on Taste | Impact on Aroma |
|---|---|---|---|
| Baking Powder | Produces light and airy texture by releasing carbon dioxide in two stages. | Neutral taste with no aftertaste, suitable for a wide range of baked goods. | Minimal aroma impact, ensuring the primary flavors dominate the final product. |
| Ammonium Carbonate | Creates crisp and porous texture, ideal for thin, dry baked items like cookies. | Can impart a slightly bitter or alkaline taste if used excessively or in moist products. | Strong ammonia aroma dissipates during baking but may linger if undercooked. |
Baking Powder versus Ammonium Carbonate: Safety and Storage
Which is safer and easier to store, baking powder or ammonium carbonate? Baking powder is generally considered safer for home use due to its stable chemical composition and longer shelf life, making it less likely to degrade or release harmful gases. Ammonium carbonate requires careful storage in airtight containers to prevent moisture absorption and decomposition, posing potential safety risks if mishandled.
Substitution Guide: When and How to Swap
Baking powder, a combination of an acid and a base, provides reliable leavening through carbon dioxide release, ideal for moist baked goods like cakes and muffins. Ammonium carbonate, also known as baker's ammonia, produces a sharper rise and crispier texture, making it best for thin, dry cookies and crackers where a clean flavor is desired. Swap ammonium carbonate in recipes that require a quick, strong rise and replace baking powder in low-moisture baked goods, but avoid using ammonium carbonate in high-moisture recipes to prevent off-flavors and uneven leavening.
Pros and Cons of Each Leavening Agent
Baking powder is a versatile leavening agent that works well at various temperatures, producing consistent rise without odor. Ammonium carbonate provides a strong leavening effect and leaves baked goods light but can release an unpleasant ammonia smell if not fully baked.
- Baking Powder Pros - Easy to use with reliable, neutral-tasting rise in cakes and cookies.
- Baking Powder Cons - Contains additives that may affect flavor slightly and is less effective in large-volume doughs.
- Ammonium Carbonate Pros - Produces crisp textures in thin crackers and cookies by releasing gas quickly.
- Ammonium Carbonate Cons - Not suitable for moist or dense baked goods due to ammonia odor risk.
Related Important Terms
Double-acting baking powder
Double-acting baking powder releases carbon dioxide gas twice during baking, providing a more controlled and sustained rise compared to ammonium carbonate, which rapidly decomposes and is best suited for dry, crisp baked goods like cookies and crackers. The chemical stability and moisture activation of double-acting baking powder make it ideal for a wider variety of recipes requiring consistent leavening and texture.
Single-acting baking powder
Single-acting baking powder releases carbon dioxide immediately upon moisture contact, providing a quick rise ideal for recipes requiring fast baking times. Ammonium carbonate, also known as baker's ammonia, produces a strong leavening effect with a distinctive crisp texture, but its ammonia odor dissipates only during thorough baking, making it less suitable for moist or dense batters.
Smelling salts (baker’s ammonia nickname)
Baking powder provides a reliable rise with a neutral taste, while ammonium carbonate, known as baker's ammonia or smelling salts, releases ammonia gas that creates a crisp texture but can leave a strong odor in dense baked goods. Ammonium carbonate is ideal for thin, dry cookies and crackers where rapid gas release enhances leavening without lingering smell, unlike baking powder which suits a wider range of recipes requiring steady, mild leavening.
Volatile alkali
Baking powder contains sodium bicarbonate and an acid, releasing carbon dioxide for leavening, while ammonium carbonate, a volatile alkali, decomposes into ammonia and carbon dioxide when heated, producing a strong rising effect but with a distinct odor. Ammonium carbonate is ideal for crisp baked goods where moisture evaporation is rapid, whereas baking powder suits a wider variety of recipes due to its neutral taste and stable rise.
Staginess (ammonium carbonate aroma)
Baking powder provides a neutral rise with minimal odor, making it ideal for moist or dense baked goods where flavor neutrality is desired. Ammonium carbonate releases a distinct ammonia aroma during baking, causing staginess that can be noticeable in thicker or less porous items, but it excels in light, dry cookies where volatilization occurs quickly.
Crispening agent
Baking powder acts as a reliable leavening agent by releasing carbon dioxide when mixed with moisture and heat, promoting even rising and light texture in baked goods. Ammonium carbonate, also known as baker's ammonia, produces crispness and a delicate crumb by releasing ammonia gas, making it ideal for thin, crisp cookies and crackers.
Thermal decomposition (leavening reaction)
Baking powder releases carbon dioxide through the thermal decomposition of sodium bicarbonate combined with acidic components, providing a controlled and sustained leavening reaction during baking. Ammonium carbonate decomposes at high temperatures into ammonia, carbon dioxide, and water vapor, producing an immediate rise ideal for low-moisture baked goods but can leave a distinctive odor if not fully dissipated.
Low-moisture leavening
Baking powder, a combination of baking soda and acid, releases carbon dioxide in moist environments for consistent rising in various batters, whereas ammonium carbonate, also known as baker's ammonia, is ideal for low-moisture, dry doughs due to its ability to produce a strong leavening effect without leaving residual taste. Ammonium carbonate rapidly decomposes into ammonia and carbon dioxide, making it perfect for thin, crispy baked goods like cookies and crackers where moisture content is minimal.
Steam-leavened texture
Baking powder releases carbon dioxide through an acid-base reaction, creating a soft, airy crumb ideal for cakes and quick breads, while ammonium carbonate decomposes into ammonia and carbon dioxide when heated, producing intense steam leavening that yields an exceptionally crisp, light texture favored in traditional cookies and crackers. Steam-leavened textures from ammonium carbonate result in a drier, flakier product due to rapid gas expansion at high temperatures, contrasting with the moist, tender rise achieved by baking powder's slower gas release.
Baking powder vs Ammonium carbonate for rising Infographic
 cookingdiff.com
cookingdiff.com