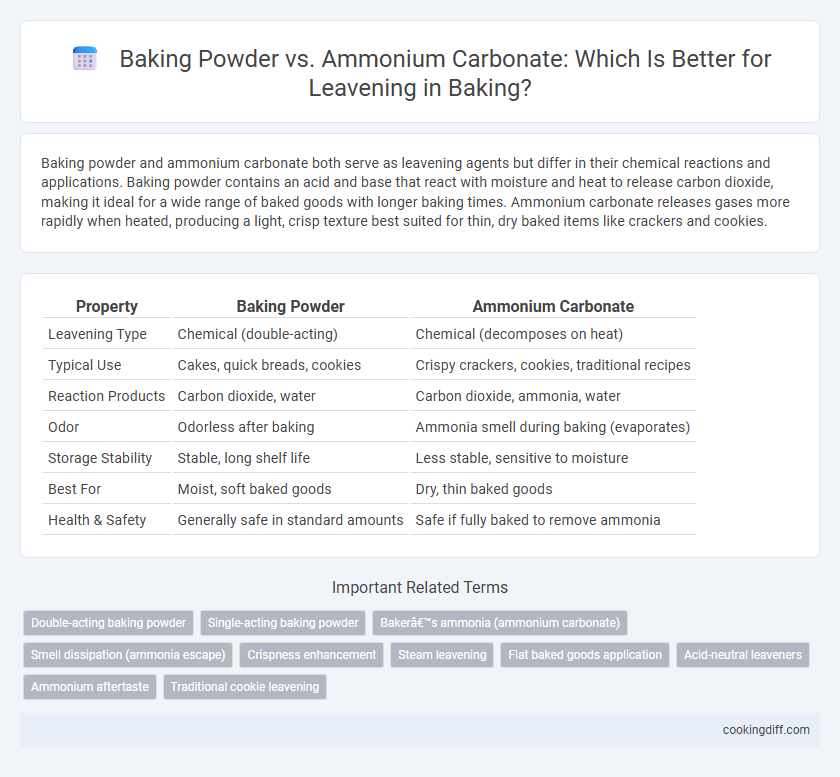
Baking powder and ammonium carbonate both serve as leavening agents but differ in their chemical reactions and applications. Baking powder contains an acid and base that react with moisture and heat to release carbon dioxide, making it ideal for a wide range of baked goods with longer baking times. Ammonium carbonate releases gases more rapidly when heated, producing a light, crisp texture best suited for thin, dry baked items like crackers and cookies.
Table of Comparison
| Property | Baking Powder | Ammonium Carbonate |
|---|---|---|
| Leavening Type | Chemical (double-acting) | Chemical (decomposes on heat) |
| Typical Use | Cakes, quick breads, cookies | Crispy crackers, cookies, traditional recipes |
| Reaction Products | Carbon dioxide, water | Carbon dioxide, ammonia, water |
| Odor | Odorless after baking | Ammonia smell during baking (evaporates) |
| Storage Stability | Stable, long shelf life | Less stable, sensitive to moisture |
| Best For | Moist, soft baked goods | Dry, thin baked goods |
| Health & Safety | Generally safe in standard amounts | Safe if fully baked to remove ammonia |
Understanding Leavening Agents: Baking Powder vs Ammonium Carbonate
Baking powder is a widely used leavening agent composed of sodium bicarbonate and an acid salt, which releases carbon dioxide when moistened and heated, helping dough rise evenly. Ammonium carbonate, also known as baker's ammonia, decomposes into ammonia, carbon dioxide, and water vapor, creating a light and crisp texture especially in cookies and crackers.
Unlike baking powder, ammonium carbonate leaves no alkaline taste but is best suited for low-moisture baked goods due to ammonia gas release. Baking powder provides consistent rise and is versatile across many types of recipes, including cakes and breads. Understanding the chemical properties and ideal applications of these agents improves texture and flavor outcomes in baking.
Chemical Composition: What Sets Baking Powder and Ammonium Carbonate Apart?
Baking powder and ammonium carbonate differ significantly in their chemical composition, affecting their leavening properties. Baking powder contains a combination of an acid (often cream of tartar) and a base (usually sodium bicarbonate), while ammonium carbonate is a single compound known as baker's ammonia.
- Baking Powder Composition - Comprised of sodium bicarbonate, acid salts, and a moisture absorber for controlled release of carbon dioxide.
- Ammonium Carbonate Composition - Made solely of ammonium bicarbonate and ammonium carbamate, which release ammonia gas and carbon dioxide when heated.
- Leavening Mechanism - Baking powder reacts in two stages, once when wet and again when heated, whereas ammonium carbonate decomposes immediately under heat.
The distinct chemical makeups result in different applications, with baking powder favored for moisture-rich batters and ammonium carbonate preferred for dry, crisp baked goods.
Leavening Mechanisms: How Each Agent Works in Baking
Baking powder releases carbon dioxide through an acid-base reaction when moistened and heated, creating bubbles that cause dough to rise. This process provides a consistent and controlled leavening effect suitable for a wide range of baked goods.
Ammonium carbonate decomposes upon heating, releasing ammonia gas and carbon dioxide, which rapidly expand dough and produce a light, crisp texture. It is preferred in traditional recipes like cookies and crackers where a strong, quick leavening action without residual acidity is desired.
Flavor Impact: Taste Differences in Baked Goods
Baking powder imparts a neutral taste with a slight acidity that blends seamlessly into most baked goods, while ammonium carbonate adds a distinctive, slightly alkaline flavor often described as sharper or more pungent. The choice between these leavening agents can significantly influence the overall flavor profile, especially in delicate recipes like cookies and crackers.
- Neutral Flavor of Baking Powder - Baking powder typically produces a mild, slightly tangy taste that does not overpower other ingredients.
- Ammonium Carbonate's Distinctive Taste - Ammonium carbonate releases ammonia, which can impart a noticeable, sharper flavor if not fully baked out.
- Recipe Compatibility - Baking powder is preferred for moist baked goods, while ammonium carbonate suits dry, thin items where its flavor disperses effectively.
Application Suitability: When to Use Baking Powder or Ammonium Carbonate
Baking powder is ideal for recipes requiring a light, airy texture, such as cakes and muffins, due to its balanced release of carbon dioxide at different temperatures. Ammonium carbonate is best suited for thin, dry baked goods like cookies and crackers where rapid gas release and moisture evaporation occur quickly. Choosing the correct leavening agent depends on the moisture content and desired texture of the final product.
Temperature Sensitivity: Baking Performance Under Heat
| Leavening Agent | Temperature Sensitivity | Baking Performance Under Heat |
| Baking Powder | Stable across a wide range of temperatures; activates progressively during baking | Produces consistent rise and crumb structure in cakes and cookies at typical oven temperatures (175degC-200degC) |
| Ammonium Carbonate | Highly temperature-sensitive; decomposes rapidly above 60degC releasing ammonia and carbon dioxide | Ideal for thin, dry baked goods; delivers quick leavening but can cause off-flavors if baking temperature is too low or uneven |
Texture Results: Comparing Crumb and Volume
How do baking powder and ammonium carbonate affect the texture of baked goods? Baking powder typically produces a finer crumb and consistent volume due to its controlled release of carbon dioxide. Ammonium carbonate creates a lighter, airier texture with a more open crumb structure but can sometimes result in uneven volume if not properly measured.
Health and Safety Considerations in Baking
Baking powder is generally safer for home baking due to its predictable reaction and lower risk of producing harmful gases, while ammonium carbonate releases ammonia that can be irritating if not fully baked out. Proper ventilation and complete baking are essential when using ammonium carbonate to avoid respiratory discomfort and ensure food safety. Consumers should choose baking powder for routine use, especially in recipes requiring longer bake times to minimize health risks associated with ammonium carbonate.
Recipe Adaptations: Substituting Baking Powder and Ammonium Carbonate
Baking powder and ammonium carbonate serve distinct roles in leavening, requiring careful recipe adjustments when substituting. Ammonium carbonate is ideal for thin, dry baked goods, while baking powder suits a broader range of cakes and muffins.
- Substitution Ratio - Use about half the quantity of ammonium carbonate compared to baking powder due to its stronger rising effect.
- Moisture Consideration - Ammonium carbonate releases ammonia, which evaporates best in low-moisture recipes to avoid off-flavors.
- Texture Impact - Baking powder produces a finer crumb, whereas ammonium carbonate results in a crispier texture in finished products.
Related Important Terms
Double-acting baking powder
Double-acting baking powder contains two types of acids that release carbon dioxide gas at different stages: once when mixed with wet ingredients and again when exposed to heat, providing a more controlled and prolonged leavening effect. Ammonium carbonate, traditionally used in European baking, rapidly decomposes into gases upon heating, ideal for thin, dry baked goods but less effective for moist or thick batters compared to double-acting baking powder.
Single-acting baking powder
Single-acting baking powder releases carbon dioxide upon moisture contact, providing immediate leavening in baked goods, whereas ammonium carbonate, also known as baker's ammonia, produces gas when heated, creating a lighter texture ideal for crisp cookies and crackers. Unlike double-acting powders, single-acting baking powder requires prompt baking after mixing to maximize its leavening effect, while ammonium carbonate decomposes fully during baking, leaving no residue.
Baker’s ammonia (ammonium carbonate)
Baker's ammonia, or ammonium carbonate, releases ammonia gas and carbon dioxide when heated, producing a rapid and strong leavening effect ideal for thin, crispy baked goods like cookies and crackers. Unlike baking powder, ammonium carbonate leaves no alkaline or metallic aftertaste, but it is unsuitable for moist or dense doughs due to ammonia gas retention.
Smell dissipation (ammonia escape)
Ammonium carbonate releases ammonia gas during baking which can cause an unpleasant smell that typically dissipates quickly at high temperatures, making it ideal for thin, dry baked goods. Baking powder, on the other hand, produces carbon dioxide without any odor, ensuring no smell escape and a neutral aroma in a wider variety of baked items.
Crispness enhancement
Baking powder releases carbon dioxide through an acid-base reaction, creating a tender, slightly raised crumb, while ammonium carbonate decomposes into gases at high temperatures, producing an exceptional crispness ideal for thin cookies and crackers. The rapid gas release and absence of moisture from ammonium carbonate result in a drier, crunchier texture compared to the softer lift provided by baking powder.
Steam leavening
Baking powder releases carbon dioxide through an acid-base reaction, providing consistent leavening, while ammonium carbonate generates ammonia and carbon dioxide upon heating, producing steam that lightens baked goods. Steam leavening from ammonium carbonate offers rapid expansion and crisp texture, ideal for thin pastries but less suitable for moisture-rich doughs due to ammonia's odor.
Flat baked goods application
Baking powder releases carbon dioxide gradually, providing steady leavening ideal for flat baked goods like cookies and crackers, while ammonium carbonate, also known as baker's ammonia, produces a rapid gas release resulting in crispier textures and is favored in traditional European recipes. Ammonium carbonate leaves no alkaline residue when fully baked, making it optimal for thin, dry baked goods, whereas baking powder offers a more neutral flavor and broader versatility across various recipes.
Acid-neutral leaveners
Baking powder, containing both acid and base components, provides balanced acid-neutral leavening by releasing carbon dioxide in two stages: when wet and during heating. Ammonium carbonate decomposes directly upon heating into gases without acid-base reaction, making it suitable for dry, thin baked goods but less versatile than acid-neutral baking powder.
Ammonium aftertaste
Ammonium carbonate releases ammonia gas during baking, which can leave a distinct aftertaste if not fully evaporated, making it less desirable for recipes with delicate flavors. Baking powder, containing baking soda and acid, provides a neutral flavor profile without the risk of an ammonia aftertaste, ensuring a cleaner taste in baked goods.
Baking powder vs ammonium carbonate for leavening. Infographic
 cookingdiff.com
cookingdiff.com