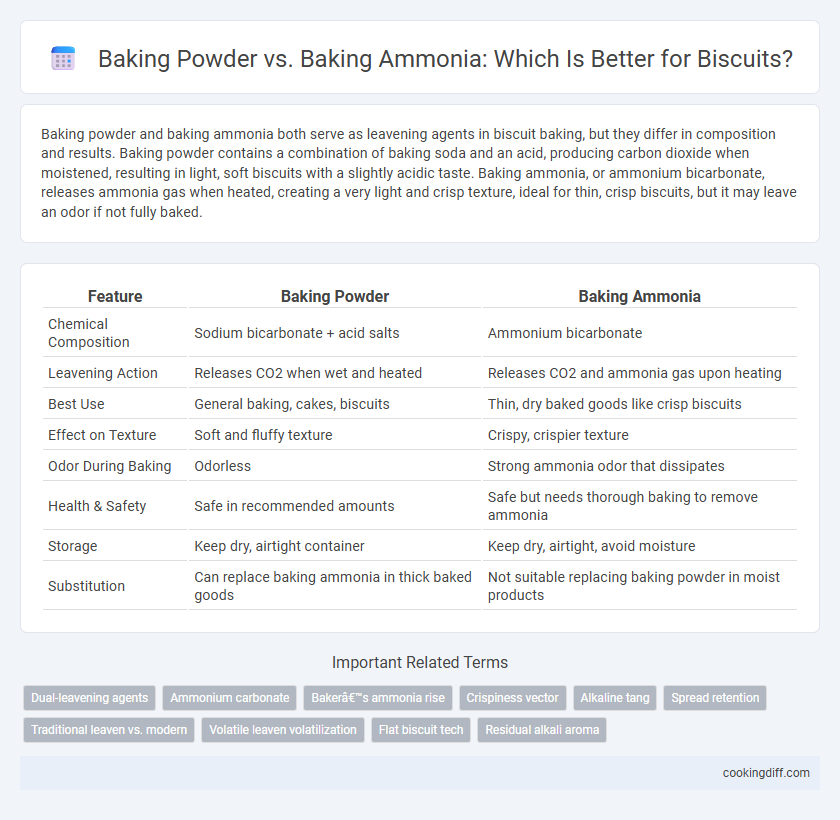
Baking powder and baking ammonia both serve as leavening agents in biscuit baking, but they differ in composition and results. Baking powder contains a combination of baking soda and an acid, producing carbon dioxide when moistened, resulting in light, soft biscuits with a slightly acidic taste. Baking ammonia, or ammonium bicarbonate, releases ammonia gas when heated, creating a very light and crisp texture, ideal for thin, crisp biscuits, but it may leave an odor if not fully baked.
Table of Comparison
| Feature | Baking Powder | Baking Ammonia |
|---|---|---|
| Chemical Composition | Sodium bicarbonate + acid salts | Ammonium bicarbonate |
| Leavening Action | Releases CO2 when wet and heated | Releases CO2 and ammonia gas upon heating |
| Best Use | General baking, cakes, biscuits | Thin, dry baked goods like crisp biscuits |
| Effect on Texture | Soft and fluffy texture | Crispy, crispier texture |
| Odor During Baking | Odorless | Strong ammonia odor that dissipates |
| Health & Safety | Safe in recommended amounts | Safe but needs thorough baking to remove ammonia |
| Storage | Keep dry, airtight container | Keep dry, airtight, avoid moisture |
| Substitution | Can replace baking ammonia in thick baked goods | Not suitable replacing baking powder in moist products |
Introduction: Understanding Baking Powder and Baking Ammonia
Baking powder is a chemical leavening agent composed of baking soda, acid, and a moisture absorber, commonly used to produce light and fluffy biscuits by releasing carbon dioxide during baking. Baking ammonia, also known as ammonium carbonate, is a traditional leavening agent that releases gases at lower temperatures, creating crispier biscuit textures without the metallic taste sometimes associated with baking soda. Understanding the differences in their chemical reactions and effects on texture helps bakers choose the appropriate leavening agent for desired biscuit results.
What Is Baking Powder?
| Baking Powder | Baking powder is a chemical leavening agent composed primarily of sodium bicarbonate, an acid salt, and a moisture absorber. It releases carbon dioxide gas when mixed with liquid and heated, causing biscuits to rise and become light and fluffy. Baking powder is preferred for biscuits due to its balanced leavening action and neutral taste compared to baking ammonia. |
What Is Baking Ammonia?
Baking ammonia, also known as ammonium bicarbonate, is a traditional leavening agent used in biscuit baking to create a light and crispy texture. It releases ammonia gas when heated, which helps the dough rise quickly without leaving a sour taste. Unlike baking powder, baking ammonia works best in thin, dry baked goods to ensure proper aeration and crispness.
Key Chemical Differences Between Baking Powder and Baking Ammonia
Baking powder contains a combination of an acid, such as cream of tartar, and a carbonate base, typically sodium bicarbonate, which react with moisture and heat to release carbon dioxide, causing dough to rise. Baking ammonia, or ammonium bicarbonate, releases carbon dioxide and ammonia gas when heated, producing a strong leavening effect but a distinct odor during baking.
Baking powder is commonly used for soft, moist baked goods like biscuits due to its controlled release of gas and neutral taste. Baking ammonia excels in thin, crispy biscuits but requires proper ventilation to dissipate ammonia fumes, highlighting its chemical volatility compared to baking powder.
Leavening Action in Biscuit Dough
Baking powder releases carbon dioxide slowly in biscuit dough, creating a light and airy texture. Baking ammonia produces gas rapidly, resulting in crispier biscuits but a distinct odor that dissipates during baking.
- Baking powder leavening - It contains both acid and base, allowing for controlled gas release during mixing and baking.
- Baking ammonia leavening - It decomposes completely at high temperatures, leaving no residue and promoting a crisp bite.
- Impact on biscuit texture - Baking powder yields softer, fluffier biscuits, whereas baking ammonia produces thinner, crisper results.
Effects on Texture: Fluffy vs. Crispy Biscuits
Baking powder produces fluffy biscuits by releasing carbon dioxide slowly, creating a light and airy texture. Baking ammonia, or ammonium bicarbonate, generates a crispier texture by evaporating completely during baking, leaving no residue.
Baking powder's leavening action helps dough rise evenly, making biscuits soft and tender. In contrast, baking ammonia's rapid gas release creates a porous, crispy structure ideal for thin, crunchy biscuits. Choosing between the two depends on the desired biscuit texture and baking time.
Flavor Impact: Taste Differences in Biscuits
Baking powder contributes a mild, slightly tangy flavor that enhances the overall taste of biscuits without overpowering other ingredients. Baking ammonia, also known as ammonium bicarbonate, produces a sharper, more distinct aroma that can impart a nostalgic, old-fashioned flavor to baked goods.
Baking ammonia releases ammonia gas during baking, which can create a unique taste that is sometimes perceived as metallic or soapy if not fully baked out. Baking powder's more neutral flavor makes it a preferred choice for delicate biscuits where a clean, balanced taste is desired.
Usage Tips: When to Choose Baking Powder or Baking Ammonia
When should you use baking powder instead of baking ammonia for biscuits? Baking powder is ideal for moist, tender biscuits as it provides a consistent rise without an ammonia odor. Baking ammonia works best for crispier, thin biscuits because it releases ammonia gas that evaporates completely, leaving no aftertaste when properly baked.
Substitution Guide: Converting Between Baking Powder and Baking Ammonia
Baking powder and baking ammonia both act as leavening agents, but they react differently when used in biscuit recipes. Understanding the correct substitution ratios and effects on texture is essential for achieving the desired biscuit quality.
- Substitution Ratio - Use 1 teaspoon of baking ammonia for every 1.5 teaspoons of baking powder to maintain leavening strength.
- Reaction Byproducts - Baking ammonia releases ammonia gas that requires thin dough for proper escape, whereas baking powder produces carbon dioxide, suitable for thicker doughs.
- Texture Impact - Baking ammonia yields crispier, lighter biscuits, while baking powder produces a softer, more cake-like crumb.
Adjustments in moisture and baking time may be necessary when substituting baking ammonia for baking powder to optimize biscuit results.
Related Important Terms
Dual-leavening agents
Baking powder and baking ammonia both serve as dual-leavening agents in biscuits, with baking powder providing a balanced, mild lift through a combination of acid and base releasing carbon dioxide, while baking ammonia, or ammonium bicarbonate, produces a sharper rise and crisper texture due to its rapid gas release and complete decomposition during baking. The choice between these agents affects biscuit texture and flavor, with baking ammonia favored in traditional recipes for its ability to create light, airy biscuits without leaving any chemical aftertaste.
Ammonium carbonate
Ammonium carbonate, commonly known as baking ammonia, provides superior leavening for biscuits by releasing carbon dioxide and ammonia gas, creating a crisp texture and lighter crumb compared to baking powder. Unlike baking powder, ammonium carbonate leaves no alkaline taste if baked thoroughly, making it ideal for thin, airy biscuits and traditional recipes.
Baker’s ammonia rise
Baker's ammonia, also known as ammonium bicarbonate, produces a strong rise in biscuits by releasing ammonia gas during baking, which creates a light, crisp texture without leaving any bitter taste. Unlike baking powder, which relies on acid-base reactions, Baker's ammonia works best in low-moisture, thin baked goods like biscuits, ensuring an optimal rise and delicate crumb structure.
Crispiness vector
Baking powder produces a balanced rise in biscuits with moderate crispiness due to its combination of acid and base components releasing carbon dioxide gradually. Baking ammonia, or ammonium bicarbonate, creates a distinctly crisp and crunchy texture by releasing gases rapidly and completely at high temperatures, making it ideal for thin, dry biscuits.
Alkaline tang
Baking powder produces a mild alkaline tang ideal for light, fluffy biscuits with a slightly neutral flavor, while baking ammonia, or ammonium carbonate, yields a stronger alkaline tang that creates crispier, more porous biscuits with a distinctive sharpness. The choice between baking powder and baking ammonia directly influences biscuit texture and flavor profile due to their differing alkaline strengths and gas release mechanisms.
Spread retention
Baking powder provides consistent spread retention in biscuits by releasing carbon dioxide slowly during baking, resulting in evenly risen and tender textures. Baking ammonia, while promoting crispiness, causes excessive spread and can lead to uneven biscuit shapes due to rapid gas release and moisture evaporation.
Traditional leaven vs. modern
Baking powder, a modern leavening agent combining acids and alkali, produces consistent rise and tender texture in biscuits through controlled CO2 release, while baking ammonia, a traditional leaven made from ammonium bicarbonate, yields a crispier, lighter biscuit with a distinct aroma but requires careful high-heat baking to avoid residual ammonia taste. Traditional baking ammonia thrives in low-moisture, thin baked goods like biscuits, whereas baking powder offers versatility and ease for a wide range of recipes.
Volatile leaven volatilization
Baking powder releases carbon dioxide through a double-acting acid-base reaction, creating a controlled leavening effect that results in light, tender biscuits. Baking ammonia (ammonium bicarbonate) volatilizes completely during baking, producing a crisp texture by releasing ammonia gas that evaporates, but it requires proper heat and ventilation to avoid residual odor.
Flat biscuit tech
Baking powder, a combination of sodium bicarbonate and acid salts, produces carbon dioxide slowly, ensuring consistent leavening and a tender texture in flat biscuits. Baking ammonia (ammonium bicarbonate) releases gas rapidly and imparts an exceptionally crisp surface but requires precise temperature control to avoid ammonia odor in thin, flat biscuit recipes.
Baking powder vs Baking ammonia for biscuits. Infographic
 cookingdiff.com
cookingdiff.com